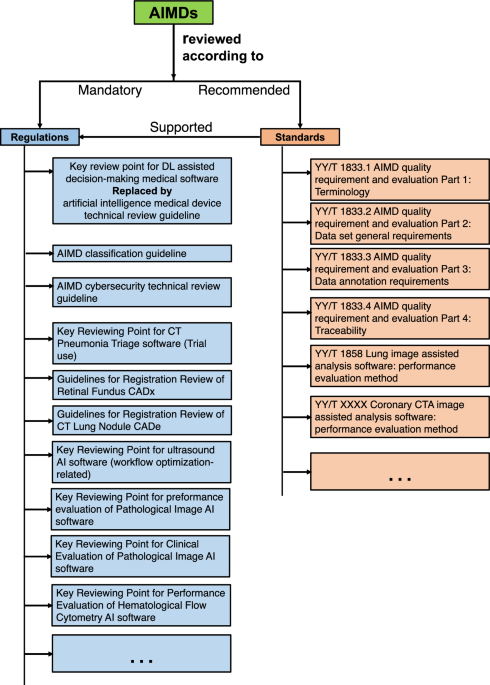
This paper focuses on how regulatory bodies respond to artificial intelligence (AI)-enabled medical devices. To achieve this, we present a comparative overview of the United States (USA), European Union (EU), and China. Our search in the governmental database identified 59 AI medical devices approved in China as of July 2023. In comparison to the rules-based regulatory approach in China, the approaches in the USA and EU are more standards-oriented.
The resurgence of interest in deep learning […]
Regulatory responses and approval status of artificial intelligence medical devices with a … – Nature
NewsChest Technology site